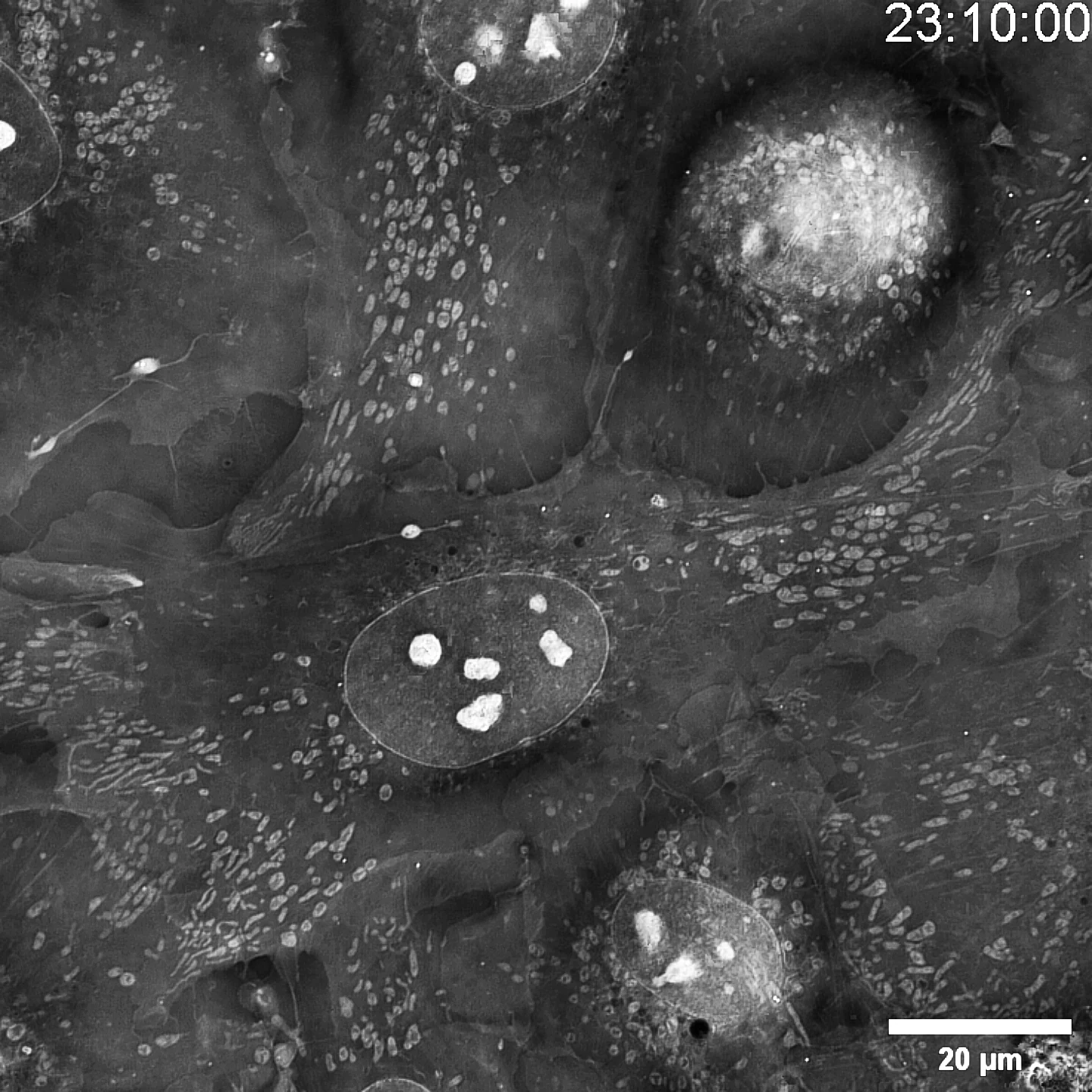
Label-free live cell imaging
Intensity diffraction tomography (IDT) technology is used, with the internal refractive index of cells as the "endogenous dye".
3D real-time super-resolution
Able to achieve super-resolution real-time imaging in three-dimensional space, capturing fine details of microscopic structures such as cells and tissues
Quantitative data analysis
By accurately measuring and statistically analyzing data, we can reveal the patterns and trends behind the data, providing a strong basis for scientific decision-making.
One-touch switching of all objective lenses without marking
4X\10X\20X\40X\100X objective lenses can all be used for real-time observation without marking, and can be switched electrically with one button.
three dimensional chromatography
The accuracy of three-dimensional chromatography is ±20nm.
Continuous imaging for weeks
Equipped with a living cell culture device, the temperature setting accuracy is ±0.1°C, the temperature setting range is ~40°C, it has an automatic gas mixing function, and supports 100% carbon dioxide gas.
Multimodal imaging
Intensity diffraction tomography (IDT), brightfield imaging, 4-channel widefield fluorescence imaging; (can also be combined with DIC/phase contrast and other imaging modes)
Well plate-level high-throughput acquisition
It can be adapted to various conventional containers such as 35mm confocal dishes and ZIRCON multi-well plates.
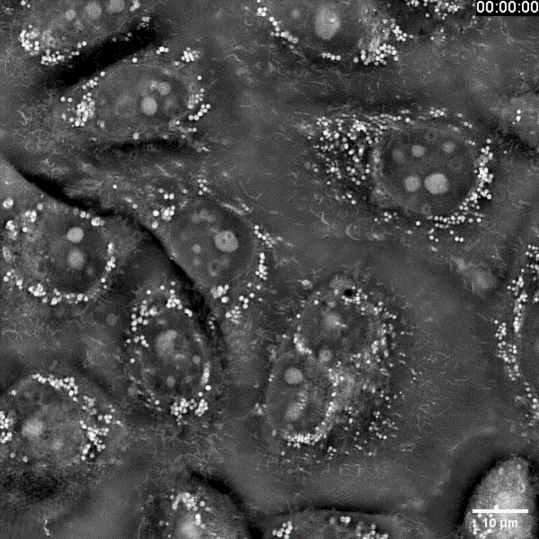
Probing sub-cellular life dynamics
You want to watch sub-cellular life in motion—how ultrafine architectures evolve over time—yet your conventional system hits a wall: too much invasive light and the specimen is gone. SC3000 turns the problem inside-out. Using Intensity-Diffraction-Tomography it recasts the sample’s own refractive index as the contrast agent—no stains, no labels, no extra photons. The result is native, real-time footage of organelle-scale dynamics with virtually zero phototoxicity or bleaching, opening a genuinely non-destructive window on living biology.
Stunning 3-D tomographic capability
Capture 100 focal planes in a single shot—160 nm axial pitch—for true 3-D diffraction tomography. Watch living cells, sub-cellular features and organelles evolve over time, switching instantly between 2-D and 3-D views without ever touching the specimen.
Fast, zero-phototoxicity sample locating
With integrated transmitted-LED and oblique-illumination detection, locating your sample is effortless and completely free of phototoxicity.
Use standard sample holders
no protocol changes required. Observe live specimens directly in the same chambers you already use for confocal microscopy.
Multi-site & long-term imaging capability
Program multiple regions of interest across the dish; then walk away. “Cell-tracking” and “Auto-lock-focusing” keep every selected site perfectly in plane for hours or days, delivering drift-free, multi-dimensional image series that maximize throughput and minimize hands-on time.
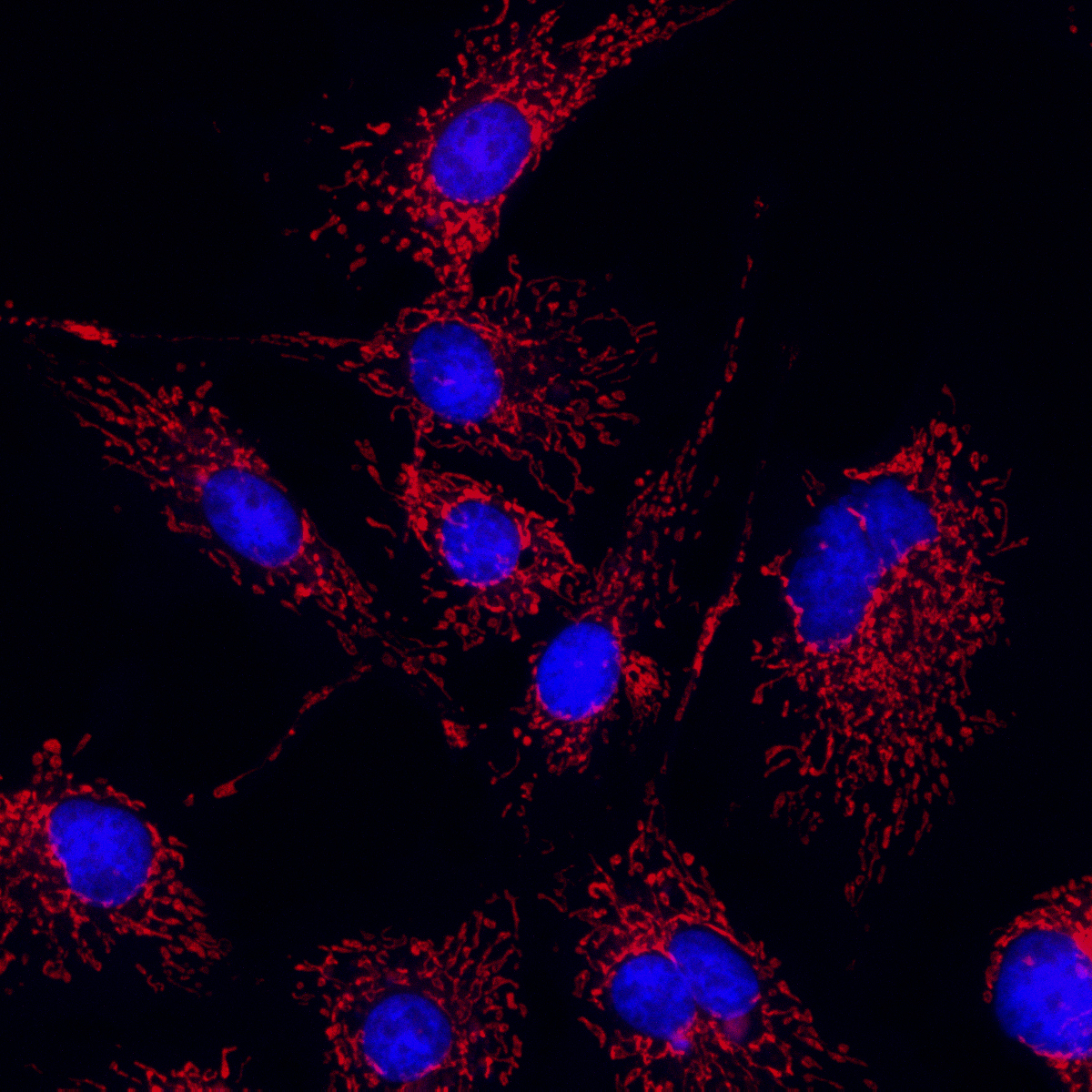
With just a few clicks, blur-free, crystal-clear fluorescence images are instantly yours.
Adaptive Fluorescence Deconvolution
Combine label-free IDT with multiplexed wide-field fluorescence in a single sweep: record the entire, uninterrupted life-story of your specimen without a stain, then trigger fluorescence at the exact moment a key event occurs—capturing both the full kinetic arc and the specific molecular signature.
This achievement has received positive reviews from C. Yang and A. Ozcan, both members of the National Academy of Inventors; R. Weissleder, a member of both the American and German Academies; M. Padget, a member of the Royal Society of Edinburgh; and Professor C. J. R. Sheppard, one of the founders of confocal microscopy theory. Citations have been published in prestigious journals such as Nature, Nature Photonics, and Nature Reviews Physics. It has also been successfully applied to high-resolution electron microscopy diffraction imaging and multiphoton laser 3D printing by S. M. Gruner, a member of the American Academy of Arts and Sciences, and M. Wegener, a member of the German Academy of Sciences.
One of the few forward models that can elegantly describe the characteristics of partially coherent imaging". And in the paper [J. Opt. Soc. Am. A 35, 1846 (2018)] it is pointed out: "It can be seen that annular illumination has a wider spatial resolution response. An interesting and important feature is that the parabolic region of low spatial frequency disappears under annular illumination, so the imaging effect of low spatial frequency can be effectively improved. The imaginary part (phase part) of the weak object transfer function at different defocus distances has been given by Zuo et al. [40]".
C. J. R. Sheppard
One of the founders of confocal microscopy theory
“Because experimental data contain noise and other errors, fast convergence can lead to noise artifacts and degrade the reconstruction quality [48,49]. To alleviate this problem, we use a small update step size for the transmission function.”

S. M. Gruner
Fellow of the American Academy of Arts and Sciences
“This is the highest numerical aperture that can be achieved by Fourier stacking imaging to date, ~1.6[30]…”. “It goes beyond the originally proposed Fourier stacking tomography scheme and realizes high-throughput Fourier stacking diffraction tomography using dark-field illumination with a large numerical aperture[11]. “Another strategy uses annular illumination[43,56]. In this method, the illumination angle is close to the maximum acceptance angle of the objective lens, that is, the captured image is near the transition region between bright field and dark field. At this time, the phase information of the sample, especially the low-frequency phase component, can be effectively converted into intensity changes and detected.”

C. Yang
Member of the National Academy of Inventors
We still write it as a phase gradient only when the meaning of this physical quantity is consistent with the ‘generalized phase’ defined in [15].” “Reference [15] extends the light intensity transfer equation to partially coherent light fields and calls this method the generalized light intensity transfer equation, or GTIE for short.”
G. K. Rohde
Professor at University of Virginia
"In 2020, Zuo Chao and his colleagues from Nanjing University of Science and Technology pushed the 3D imaging technology based on the Fourier stacking principle to beyond proof-of-principle demonstration... and achieved 3D object reconstruction without axial defocus stacking."

M Guizar-Sicairos与P. Thibault
Founder of stacked imaging
The required (phase) information can also be obtained by recording multiple holograms of the sample at different camera distances, different illumination wavelengths [38], or by illuminating the sample at different angles. In the paper [Opt. Express 25, 4438 (2017)] it is pointed out that: "This multi-angle illumination can also be used to perform depth tomography in a certain space [22-24]".

M. Unser
Member of the Swiss National Academy of Engineering Sciences
“Generally, in phase microscopy, the use of annular illumination has been found to provide higher resolution, as the lateral resolution is extended to twice the coherent diffraction limit [32,33,34,35]. In intensity-transfer quantitative phase imaging, in addition to improving resolution, annular illumination can also reduce the required measurement data [36]. Other advantages include shorter and lower-intensity exposures and compatibility with conventional microscopy techniques, as coherent illumination is not required [36]”.

T. K. Gaylord
Georgia Institute of Technology Chair Professor
This is the largest numerical aperture for illumination ever achieved."

C. F. Kaminski
Director of the EPSRC Research Centre at the University of Cambridge
Two papers and two original images from our team were cited in Nature [Nature 637, 281 (2025)], and the paper commented: "...exceeds the capabilities of conventional quantitative phase imaging techniques [41]. A key advantage of this method is its ability to capture a three-dimensional volume of 1.7×1010 voxels containing 2×104 cells, with a field of view of 1.77 mm2, without the need for sample tilt or axial defocus."

J. Miao
One of the founders of the field of coherent diffraction imaging
.png?w=3072&fmt=webp)